Transforming 300+
clinical teams
Over nine months, we helped Novartis embed new ways of working across hundreds of clinical trial teams to empower innovation across the organisation.

250+
workshops and sessions run
600+
people adopted new ways of working
13
&us transformation coaches involved
About Novartis Global Clinical Operations
Novartis is under intense pressure to grow in the face of increasing competition. To ensure growth and stability, Novartis relies on its Global Clinical Operations (GCO) division to deliver innovative new drugs and therapies to the market. The 300+ clinical trial teams within this division conduct rigorous scientific research to ensure new medicines are safe, effective and meet relevant ethical and regulatory frameworks. However, these teams were siloed and are spread across several countries (China, Japan, Switzerland, Ireland and the U.S.A.), posing distinct challenges to Novartis – from speed of innovation to cross-team collaboration.
Activating new ways of working
The GCO division had been on a journey of transformation over several years, and sought to define best practices, guidance for leaders and new ways of working. These were defined through the creation of a Centre of Excellence and a playbook for clinical trial teams to codify best practice behaviours and processes. By doing this, the ultimate goal was to ensure Novartis remained at the forefront of pharmaceutical innovation, with clinical trial teams that could operate at an accelerated pace, and who could adapt to change as it emerged.
However, the playbook required a steep adoption curve and teams were unclear on how to bring the theory into practice in their day-to-day life. Our priority was therefore to design and roll out learning experiences that would successfully embed the playbook learnings into every team, with hands-on coaching alongside.
The big question Novartis GCO set us:
How do new ways of working become an active, lived reality across clinical trial teams, in order to accelerate their commercial impact and help innovation flow?
Our approach
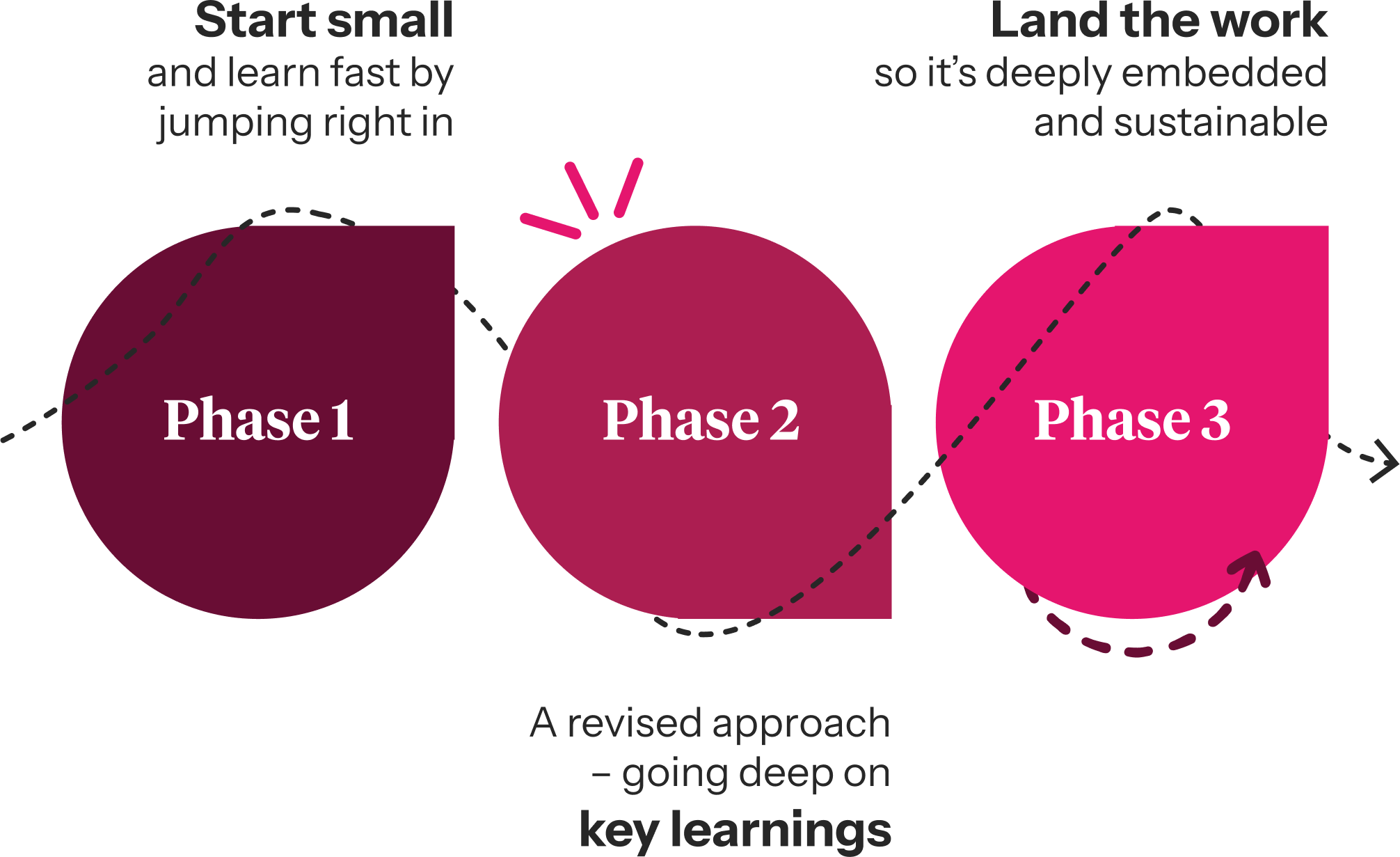
Phase 1 – start small and learn fast
The focus for phase one was to bring the playbook to life for 100 teams – a subset of the clinical trial teams, which collectively number over 300 individual teams.
To begin, we embedded several of our transformation practitioners directly into the teams, so that they could fully immerse themselves in the teams' day-to-day. Having context for how the teams worked, we then started bridging the gap between the playbook and how these behaviours could be used in practice.
Inspired by Nature, we encouraged a shift towards an ‘ecosystem mindset’ and amplified the new ways of working.
-min.png?width=2134&height=2214&name=Group%2043027%20(1)-min.png)
We supported these teams across several geographies, inserting our coaches into their daily work and helping refine their existing meetings and cadence into a focused and sustainable rhythm. Part of this was coaching the team to define clear meeting intent and separating meetings that focused on planning the work, versus doing the work. To do this, we were also supported by Novartis’ own coaching teams within the GCO division, who were tasked with coordinating the clinical trial teams transformation internally. This collaboration created a ‘coaching academy’ identity that fostered trust, transparency and improved teamwork.
“What really stood out was how fully the &us coaches immersed themselves in our team, understanding our unique processes and culture here at Novartis. We are a large, complex organisation operating in a complex environment – so that’s no easy feat.”
Working with the leaders of each team, we enabled them to:
- Establish a consistent sprint rhythm to accelerate the delivery of their clinical studies.
- Distil the playbook into five key principles, providing actionable how-to guidance on applying agile practices in their day-to-day work.
- Create space to experiment and learn, helping teams generate new insights to support their trial objectives.
Identifying the need to pivot
However, it quickly became evident that under the existing programme plan, this approach would prove slow, as more foundational learning was needed. We also realised leaders across different line functions had nuanced challenges that required tailored teaching on the new ways of working. Furthermore, individuals needed space to understand and digest the learnings from the playbook outside of daily work.
Given these challenges, continuing down the initial path of focusing on just 100 teams before scaling more broadly would require an unsustainable amount of coaching resource and time, so we quickly pivoted to determine the best way to deliver greater impact more efficiently.
Phase 2 – a revised approach
We identified that by pivoting our support to focus on clinical trial line functions instead of standalone individual clinical trial teams, we were able to have an impact across all 300+ clinical trial teams simultaneously.
We did this by designing and delivering large scale learning experiences within the line functions. These learning experiences were designed to take participants on an active transformation journey and make them the champions of the change.
Creating change champions
meant fostering:
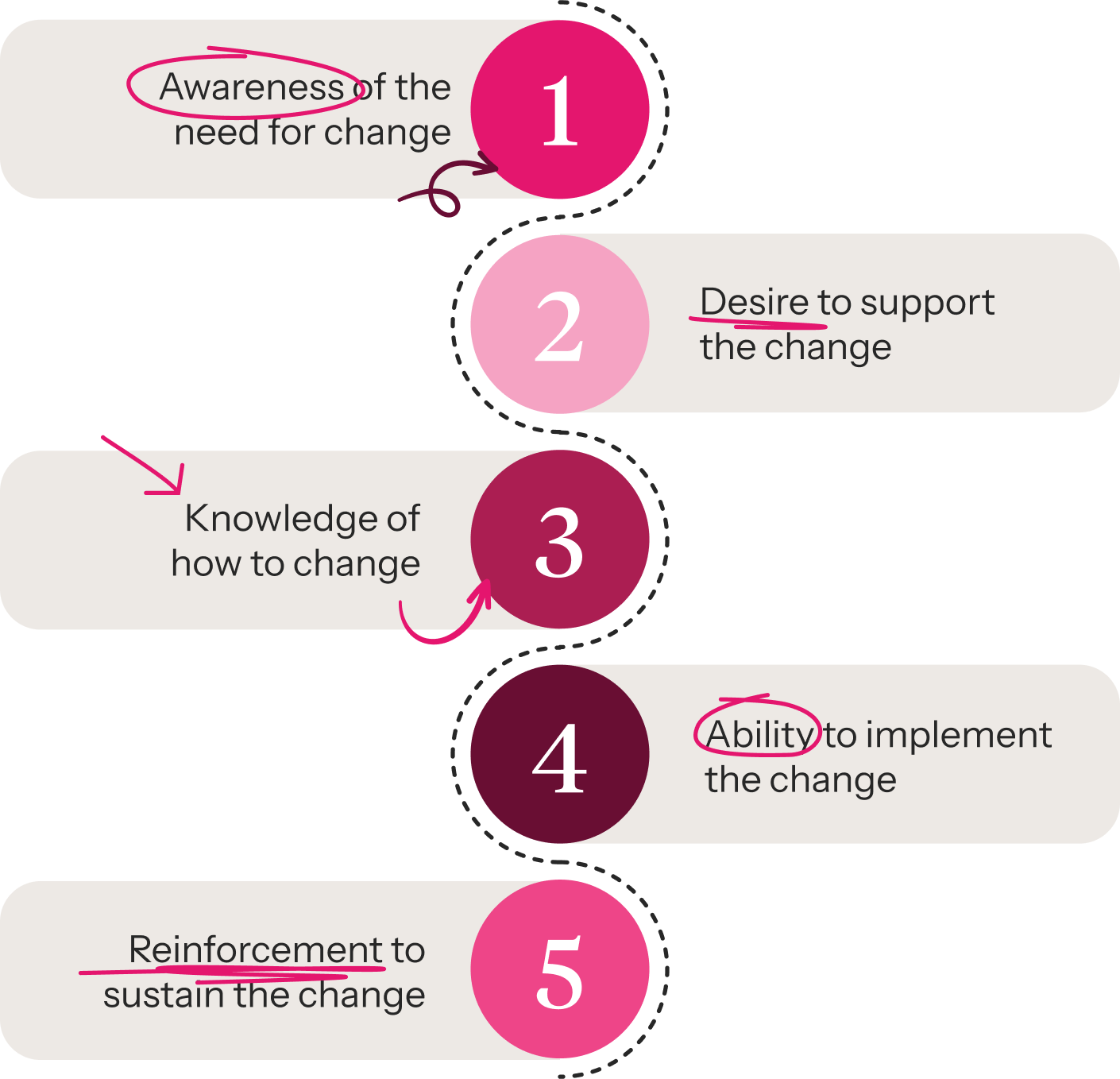
This process embedded playbook foundations rapidly, as the work was no longer happening in silos. It also created more confident community leaders (drawn from leaders of the line functions) to act as catalysts for change. This meant we were able to train and coach every core team member of the Global Clinical Operations team in a three-month period, instead of over multiple years.
“&us established a strong and close partnership with our coaching academy from the outset, collaborating closely with us to design practical coaching experiments and create a collaborative space for our teams to explore new concepts in a safe environment. They really understood our desire for a human-centric approach, and built platforms that fostered safe, collaborative, trusted, and playful exchanges. By combining the ‘what we do’ with the ‘how we do it’, they helped successfully activate our new ways of working.”
Phase 3 – land the work
The final phase had two key objectives: to continue to roll out the playbook awareness programme across the teams, and build sustainability into the system so that it could continue to move positively forward – with, or without, &us.
To achieve this, we:
- Cemented the role of community leaders with deeper training in agile and systems design, with dedicated coaching to help them acquire expansive thinking skills.
- Created a network of connected community leaders, who support each other and are positioned as owners of the change.
- Levelled-up other line functions with playbook training to bring them up to speed with those who had already received coaching.
- Equipped the GCO clinical trial teams with content and tools to support their onward journey.
Key outcomes

Embedded new behaviours in 300+ clinical teams
In just nine short months, the programme went far beyond the initial target of engaging 100 clinical trial teams and instead impacted over 300. This exceeded expectations for the programme, creating confidence in the approach and accelerating the programme’s impact on Novartis’ innovation capabilities.

Collaborative, fluid working across geographies
With the full integration of the playbook and consistent ways of working, clinical teams (including 660 individuals) are now more collaborative – helping foster stronger working relationships that are less transactional.

Empowered leaders
From community leaders to clinical team leads, leaders at Novartis are more confident practitioners of the new principles and ways of working. This positively impacts how they lead teams now – and in the future.
The work, however, is just getting started. Our current focus is helping Novartis leverage the insights and learnings from the initial three phases to build the business case for continued and sustained investment in the programme over a multi-year term.